第三代长效升白针——艾贝格司亭α,打好化疗持久战的新武器
二战时,一艘载满芥子气毒气弹的轮船在意大利巴厘港爆炸,有医生发现,很多暴露于芥子气中的当地军民,其淋巴组织与骨髓的生长受到了抑制。在此基础上,药理学家Louis Goodman和Alfred Gilman从芥子气中提取出氮芥物质并证实了氮芥可有效抑制淋巴瘤的生长,从此开启了化学药物治疗肿瘤的时代。
时至今日,虽然免疫治疗、靶向治疗等新型疗法层出不穷,但化疗依然是癌症治疗的中流砥柱。而最初导致化疗诞生的骨髓抑制现象,现在也依然是化疗最常见的一类不良反应,其中又以骨髓抑制导致的中性粒细胞减少最为严重。根据中性粒细胞绝对计数,中性粒细胞减少可以分为4个等级[1]。
时至今日,虽然免疫治疗、靶向治疗等新型疗法层出不穷,但化疗依然是癌症治疗的中流砥柱。而最初导致化疗诞生的骨髓抑制现象,现在也依然是化疗最常见的一类不良反应,其中又以骨髓抑制导致的中性粒细胞减少最为严重。根据中性粒细胞绝对计数,中性粒细胞减少可以分为4个等级[1]。
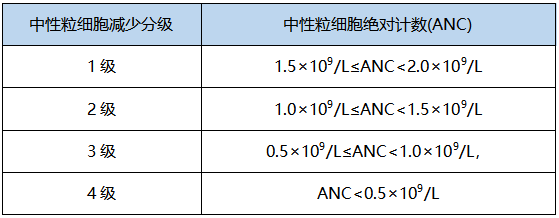
对癌症患者来说,中性粒细胞计数越低,中性粒细胞减少的持续时间越长,发生严重感染的风险也就越高[2]。为了避免严重感染等更加难以处理的情况发生,出现3级或以上中性粒细胞减少的患者往往要减少化疗剂量、推迟化疗时间,或者更换化疗方案甚至是终止化疗。
中性粒细胞计数越低,中性粒细胞减少持续时间越长,患者严重感染风险越高
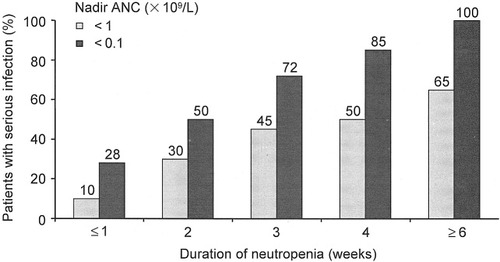
而减少化疗剂量的代价是十分惨痛的:化疗剂量越低,患者生存率越差。根据意大利一项研究,在接受CMF(环磷酰胺+甲氨蝶呤+氟尿嘧啶)辅助联合化疗方案的乳腺癌患者中[3]:
接受85%或以上计划剂量的患者,20年生存率为52%
接受65%~84%计划剂量的患者,20年生存率为32%
接受65%以下计划剂量的患者,20年生存率只有25%,与安慰剂组相似
接受85%或以上计划剂量的患者,20年生存率为52%
接受65%~84%计划剂量的患者,20年生存率为32%
接受65%以下计划剂量的患者,20年生存率只有25%,与安慰剂组相似
化疗剂量越低,患者生存率越差
为降低严重中性粒细胞减少或发热性中性粒细胞减少(FN)的发生率,保证化疗顺利完成,临床上常常会应用升白针进行干预。“升白针”的主要有效成分是粒细胞集落刺激因子(G-CSF),具有促进中性粒细胞生成和成熟的作用。“升白针”的应用使得很多化疗患者得以继续接受治疗,成为帮助患者打好化疗持久战的有力武器。
目前临床上常用的G-CSF有短效和长效两种,两者的作用原理是类似的,主要都是刺激骨髓造血,区别主要在于作用时间的长短:长效G-CSF作用时间长,每个化疗周期只需注射一次,应用更加方便;短效G-CSF药效持续时间短,需要频繁注射,注射期间要经常抽血复查。
升白针可以提前用吗?
在诞生之初,升白针主要是用来治疗已经发生严重中性粒细胞减少或FN的患者,虽说亡羊补牢为时未晚,但升白针能否提前应用以防患于未然呢?相关研究显示,在发生严重的中性粒细胞减少或FN之前,提前使用升白针来预防,可以有效降低严重中性粒细胞减少或FN的发生率[4-7]。
升白针的预防性使用可以分成两类:
针对接受高FN风险化疗方案的患者,或接受中FN风险化疗方案并伴有FN风险因素的患者,可以在首次化疗时就开始预防性使用升白针,也就是一级预防;
针对在此前化疗周期中发生过FN的患者,在后续化疗时预防性使用升白针,也就是二级预防。
相关荟萃分析显示,一级预防使用短效升白针,FN发生风险降低57%;而使用长效升白针的话,由于使用方便,患者依从性更好,效果更加明显,FN发生风险可降低73%[4]。
另外还有研究报道,一级预防使用长效G-CSF可显著降低患者中性粒细胞减少相关住院率[5] 及感染相关死亡风险[6]。二级预防的效果虽然比一级预防差一些,但也能有效降低FN的发生风险[7]。
升白针有什么副作用?
对癌症患者来说,适当的升白是助力完成治疗,获得最佳疗效的保障,但升白的副作用也需要重视。目前常见的主要不良反应包括骨痛、局部过敏反应等。
升白针主要的不良反应就是骨痛。在注射升白针以后,骨髓受刺激增殖扩张,产生的免疫细胞也会受刺激分泌一些细胞因子,造成炎症和疼痛[8]。同时,CSF还有募集炎性细胞的作用,会让周围的痛觉神经更为敏感。
在非格司亭的II期和III期临床试验中,大约有10%的患者会发生骨痛,3%的患者会发生重度骨痛[9]。对于升白针引起的骨痛,一般可以使用NSAIDs类药物止痛;NSAIDs无效的患者,可以进一步使用阿片类止痛药或萘普生、氯雷他定等抗组胺药治疗[10];有研究显示,在注射升白针当天服用抗组胺药萘普生,有助于预防升白针引起的骨痛[11]。
过敏反应也是升白针的常见不良反应,这可能与长效升白针中的聚乙二醇成分有关[12]。
目前的第二代升白针都是通过聚乙二醇包被实现G-CSF的长效化,而现代生活中,由于牙膏、洗发液等很多日化用品都含有聚乙二醇,导致很多人体内存在聚乙二醇抗体,这就使得采用聚乙二醇化技术路线的第二代长效升白针较易引发过敏。
长效升白针引起的过敏反应一般表现为皮疹、荨麻疹等,给予对症治疗即可,偶有严重过敏反应发生,多为个案报道。
因此,各位患者千万不要因为害怕骨痛、过敏反应等而拒绝升白,合理升白利大于弊。对于具有高FN风险的化疗患者而言,如果因为害怕骨痛、过敏反应而不进行升白治疗,有可能会因为中性粒细胞减少造成严重的感染,或者不得不减少治疗剂量,甚至可能造成死亡。
长效升白新选择——艾贝格司亭α
除第一代短效升白针和第二代长效升白针之外,目前第三代长效升白针艾贝格司亭α也已诞生。不同于第二代长效升白针的聚乙二醇包被技术路线,第三代的艾贝格司亭α采用Fc融合蛋白技术实现G-CSF的长效化。
目前临床上常用的G-CSF有短效和长效两种,两者的作用原理是类似的,主要都是刺激骨髓造血,区别主要在于作用时间的长短:长效G-CSF作用时间长,每个化疗周期只需注射一次,应用更加方便;短效G-CSF药效持续时间短,需要频繁注射,注射期间要经常抽血复查。
升白针可以提前用吗?
在诞生之初,升白针主要是用来治疗已经发生严重中性粒细胞减少或FN的患者,虽说亡羊补牢为时未晚,但升白针能否提前应用以防患于未然呢?相关研究显示,在发生严重的中性粒细胞减少或FN之前,提前使用升白针来预防,可以有效降低严重中性粒细胞减少或FN的发生率[4-7]。
升白针的预防性使用可以分成两类:
针对接受高FN风险化疗方案的患者,或接受中FN风险化疗方案并伴有FN风险因素的患者,可以在首次化疗时就开始预防性使用升白针,也就是一级预防;
针对在此前化疗周期中发生过FN的患者,在后续化疗时预防性使用升白针,也就是二级预防。
相关荟萃分析显示,一级预防使用短效升白针,FN发生风险降低57%;而使用长效升白针的话,由于使用方便,患者依从性更好,效果更加明显,FN发生风险可降低73%[4]。
另外还有研究报道,一级预防使用长效G-CSF可显著降低患者中性粒细胞减少相关住院率[5] 及感染相关死亡风险[6]。二级预防的效果虽然比一级预防差一些,但也能有效降低FN的发生风险[7]。
升白针有什么副作用?
对癌症患者来说,适当的升白是助力完成治疗,获得最佳疗效的保障,但升白的副作用也需要重视。目前常见的主要不良反应包括骨痛、局部过敏反应等。
升白针主要的不良反应就是骨痛。在注射升白针以后,骨髓受刺激增殖扩张,产生的免疫细胞也会受刺激分泌一些细胞因子,造成炎症和疼痛[8]。同时,CSF还有募集炎性细胞的作用,会让周围的痛觉神经更为敏感。
在非格司亭的II期和III期临床试验中,大约有10%的患者会发生骨痛,3%的患者会发生重度骨痛[9]。对于升白针引起的骨痛,一般可以使用NSAIDs类药物止痛;NSAIDs无效的患者,可以进一步使用阿片类止痛药或萘普生、氯雷他定等抗组胺药治疗[10];有研究显示,在注射升白针当天服用抗组胺药萘普生,有助于预防升白针引起的骨痛[11]。
过敏反应也是升白针的常见不良反应,这可能与长效升白针中的聚乙二醇成分有关[12]。
目前的第二代升白针都是通过聚乙二醇包被实现G-CSF的长效化,而现代生活中,由于牙膏、洗发液等很多日化用品都含有聚乙二醇,导致很多人体内存在聚乙二醇抗体,这就使得采用聚乙二醇化技术路线的第二代长效升白针较易引发过敏。
长效升白针引起的过敏反应一般表现为皮疹、荨麻疹等,给予对症治疗即可,偶有严重过敏反应发生,多为个案报道。
因此,各位患者千万不要因为害怕骨痛、过敏反应等而拒绝升白,合理升白利大于弊。对于具有高FN风险的化疗患者而言,如果因为害怕骨痛、过敏反应而不进行升白治疗,有可能会因为中性粒细胞减少造成严重的感染,或者不得不减少治疗剂量,甚至可能造成死亡。
长效升白新选择——艾贝格司亭α
除第一代短效升白针和第二代长效升白针之外,目前第三代长效升白针艾贝格司亭α也已诞生。不同于第二代长效升白针的聚乙二醇包被技术路线,第三代的艾贝格司亭α采用Fc融合蛋白技术实现G-CSF的长效化。
艾贝格司亭α预充式注射器
作为一种新型长效升白针,艾贝格司亭α有着不弱于第一代短效升白针非格司亭和第二代长效升白针培非格司亭的升白效果,同时安全性更为出众 [13,14]:
相比于非格司亭,艾贝格司亭α的骨痛发生率更低,使用更为便捷,患者依从性更好;
相比于培非格司亭,艾贝格司亭α不含具有致敏性的聚乙二醇、吐温80等成分,过敏反应的发生率更低。
在今年5月9日,艾贝格司亭α获批上市,为癌症患者打好化疗持久战提供了有力的新武器,帮助癌症患者进一步减轻化疗期间的身体负担。期待这一新型长效升白针能够为更多肿瘤患者长期生存保驾护航。
参考文献:
1.中国临床肿瘤学会指南工作委员会.中国临床肿瘤学会(CSCO)肿瘤放化疗相关中性粒细胞减少症规范化管理指南(2021)[J].临床肿瘤学杂志, 2021, 026(007):638-648.
2.Crawford J, Dale D C, Lyman G H. Chemotherapy‐induced neutropenia: risks, consequences, and new directions for its management[J]. Cancer, 2004, 100(2): 228-237.
3.Bonadonna G, Valagussa P, Moliterni A, et al. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer—the results of 20 years of follow-up[J]. New England Journal of Medicine, 1995, 332(14): 901-906.
4.Wang L, Baser O, Kutikova L, Page JH, Barron R. The impact of primary prophylaxis with granulocyte colony-stimulating factors on febrile neutropenia during chemotherapy: a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer. 2015 Nov;23(11):3131-40.
5.Naeim A, Henk HJ, Becker L, Chia V, Badre S, Li X, Deeter R. Pegfilgrastim prophylaxis is associated with a lower risk of hospitalization of cancer patients than filgrastim prophylaxis: a retrospective United States claims analysis of granulocyte colony-stimulating factors (G-CSF). BMC Cancer. 2013 Jan 8;13:11.
6.Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007 Jul 20;25(21):3158-67.
7.李惠平,樊征夫,郑虹,高雨农,涂梅峰,宋国红,邵彬,高天,朱军.聚乙二醇化重组人粒细胞集落刺激因子初级与次级预防化疗后中性粒细胞减少的有效性和安全性临床研究[J].中国肿瘤临床,2019,46(14):739-744
8.Lambertini M, Del Mastro L, Bellodi A, et al. The five “Ws” for bone pain due to the administration of granulocyte-colony stimulating factors (G-CSFs)[J]. Critical reviews in oncology/hematology, 2014, 89(1): 112-128.
for bone pain due to the administration of granulocyte-colony stimulating factors (G-CSFs)[J]. Critical reviews in oncology/hematology, 2014, 89(1): 112-128.
9.Renwick W, Pettengell R, Green M. Use of filgrastim and pegfilgrastim to support delivery of chemotherapy: twenty years of clinical experience[J]. BioDrugs, 2009, 23: 175-186.
10.Tsuboi S, Hayama T, Miura K, et al. Higher incidence of pegfilgrastim-induced bone pain in younger patients receiving myelosuppressive chemotherapy: a real-world experience[J]. Journal of Pharmaceutical Health Care and Sciences, 2023, 9(1): 1-5.
11.Kirshner J J, Heckler C E, Dakhil S R, et al. Prevention of pegfilgrastim-induced bone pain (PIP): A URCC CCOP randomized, double-blind, placebo-controlled trial of 510 cancer patients[J]. Journal of Clinical Oncology, 2010, 28(15_suppl): 9014-9014.
12.Neumann T A, Foote M A. The safety profile of filgrastim and pegfilgrastim[J]. Twenty Years of G-CSF: Clinical and Nonclinical Discoveries, 2012: 395-408.
13.Daley W, Shao Z, Zhang Q, et al. Abstract P5-16-14: A randomized, multicenter phase III study of once-per-cycle administration of efbemalenograstim alfa (F-627), a novel long-acting dimeric rhG-CSF, for prophylaxis of chemotherapy-induced neutropenia in patients with breast cancer[J]. Cancer Research, 2022, 82(4_Supplement): P5-16-14-P5-16-14.
14.Glaspy J, Daley W, Bondarenko I, et al. A Phase III, Randomized, Multi-Center, Open-Label, Fixed Dose, Neulasta Active-Controlled Clinical Trial of F-627, a Novel G-CSF, in Women with Breast Cancer Receiving Myelotoxic Chemotherapy[J]. Blood, 2021, 138: 4290.
相比于非格司亭,艾贝格司亭α的骨痛发生率更低,使用更为便捷,患者依从性更好;
相比于培非格司亭,艾贝格司亭α不含具有致敏性的聚乙二醇、吐温80等成分,过敏反应的发生率更低。
在今年5月9日,艾贝格司亭α获批上市,为癌症患者打好化疗持久战提供了有力的新武器,帮助癌症患者进一步减轻化疗期间的身体负担。期待这一新型长效升白针能够为更多肿瘤患者长期生存保驾护航。
参考文献:
1.中国临床肿瘤学会指南工作委员会.中国临床肿瘤学会(CSCO)肿瘤放化疗相关中性粒细胞减少症规范化管理指南(2021)[J].临床肿瘤学杂志, 2021, 026(007):638-648.
2.Crawford J, Dale D C, Lyman G H. Chemotherapy‐induced neutropenia: risks, consequences, and new directions for its management[J]. Cancer, 2004, 100(2): 228-237.
3.Bonadonna G, Valagussa P, Moliterni A, et al. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer—the results of 20 years of follow-up[J]. New England Journal of Medicine, 1995, 332(14): 901-906.
4.Wang L, Baser O, Kutikova L, Page JH, Barron R. The impact of primary prophylaxis with granulocyte colony-stimulating factors on febrile neutropenia during chemotherapy: a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer. 2015 Nov;23(11):3131-40.
5.Naeim A, Henk HJ, Becker L, Chia V, Badre S, Li X, Deeter R. Pegfilgrastim prophylaxis is associated with a lower risk of hospitalization of cancer patients than filgrastim prophylaxis: a retrospective United States claims analysis of granulocyte colony-stimulating factors (G-CSF). BMC Cancer. 2013 Jan 8;13:11.
6.Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007 Jul 20;25(21):3158-67.
7.李惠平,樊征夫,郑虹,高雨农,涂梅峰,宋国红,邵彬,高天,朱军.聚乙二醇化重组人粒细胞集落刺激因子初级与次级预防化疗后中性粒细胞减少的有效性和安全性临床研究[J].中国肿瘤临床,2019,46(14):739-744
8.Lambertini M, Del Mastro L, Bellodi A, et al. The five “Ws”
9.Renwick W, Pettengell R, Green M. Use of filgrastim and pegfilgrastim to support delivery of chemotherapy: twenty years of clinical experience[J]. BioDrugs, 2009, 23: 175-186.
10.Tsuboi S, Hayama T, Miura K, et al. Higher incidence of pegfilgrastim-induced bone pain in younger patients receiving myelosuppressive chemotherapy: a real-world experience[J]. Journal of Pharmaceutical Health Care and Sciences, 2023, 9(1): 1-5.
11.Kirshner J J, Heckler C E, Dakhil S R, et al. Prevention of pegfilgrastim-induced bone pain (PIP): A URCC CCOP randomized, double-blind, placebo-controlled trial of 510 cancer patients[J]. Journal of Clinical Oncology, 2010, 28(15_suppl): 9014-9014.
12.Neumann T A, Foote M A. The safety profile of filgrastim and pegfilgrastim[J]. Twenty Years of G-CSF: Clinical and Nonclinical Discoveries, 2012: 395-408.
13.Daley W, Shao Z, Zhang Q, et al. Abstract P5-16-14: A randomized, multicenter phase III study of once-per-cycle administration of efbemalenograstim alfa (F-627), a novel long-acting dimeric rhG-CSF, for prophylaxis of chemotherapy-induced neutropenia in patients with breast cancer[J]. Cancer Research, 2022, 82(4_Supplement): P5-16-14-P5-16-14.
14.Glaspy J, Daley W, Bondarenko I, et al. A Phase III, Randomized, Multi-Center, Open-Label, Fixed Dose, Neulasta Active-Controlled Clinical Trial of F-627, a Novel G-CSF, in Women with Breast Cancer Receiving Myelotoxic Chemotherapy[J]. Blood, 2021, 138: 4290.




